Strattice™ Reconstructive Tissue Matrix
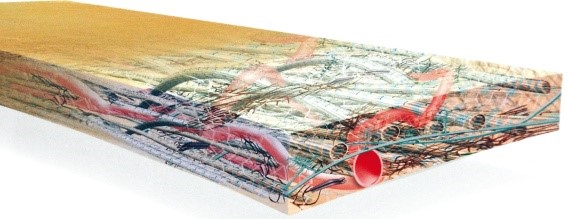
Preservation of intact extracellular matrix is critical to support regeneration.
Strattice™ Reconstructive Tissue Matrix is obtained from pig skin and undergoes a non-damaging, patented procedure. In doing so, cells are removed and the major component believed to play a major role in the xenogenic rejection reaction (alpha-1,3-Gal epitope) is significantly reduced.
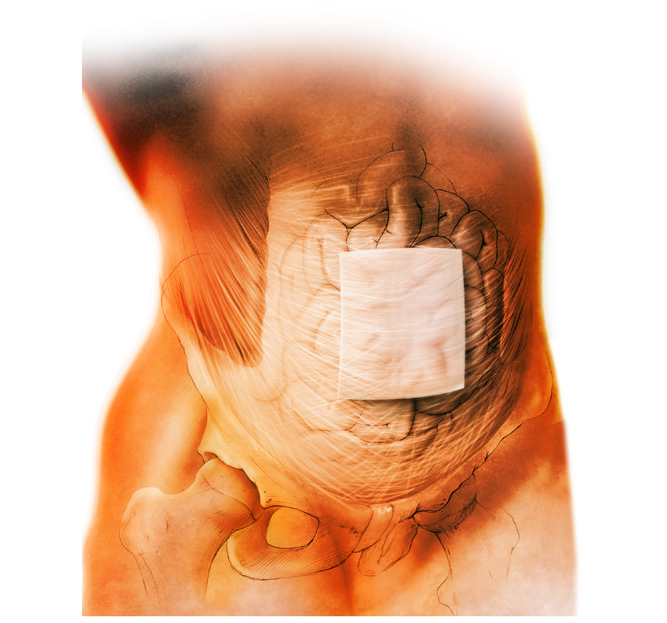
This matrix has been shown to provide final single-phase reconstruction in the presence of contamination or infection.3,5,6
Benefits:
- Enables rapid revascularization, cell repopulation and white blood cell migration (1)
- Allows local treatment in case of infection and exposure of the implant (3)
- Implant adhesion is minimized (4)
- Offers strong reconstruction thanks to excellent biomechanical strength (1,2)
Strattice™ Reconstructive Tissue Matrix offers you a clinically proven option for treating patients with complex abdominal wall reconstructions.
Strattice™ Reconstructive Tissue Matrix showed a low recurrence rate and no graft removal in complex abdominal wall reconstructions, (3) allowing for a safe and effective alternative to multi-stage reconstruction adhesion and without the complications associated with synthetic mesh.
References:
- Connor J., et al, Retention of structural and biochemical integrity in a biologic mesh supports tissue remodeling in a primate abdominal wall model. Regen Med.2009;4(2): 185-195
- Laboratoriumgegevens beschikbaar
- Itani K, et al. Prospective Clinical Study Evidence of Safe, Single-Stage Repair of Infected/ Contaminated Abdominal Incisional Hernias Using Strattice Reconstructive Tissue Matrix. Hernia. 2009;13(1):S1-S32):S28
- Burns NK, et al. Non-cross linked porcine acellular dermal matrices for abdominal wall reconstruction. Plast Reconstr Surg. 2009:PMID:19910855
- Skipworth JRA, et al. Mesh repair of complex, incisional hernias, utilizing soft tissue reconstruction & biological mesh insertion: a consecutive, single-team experience. Hernia (2011) 15 (Suppl 2): S37-S66, P066
- McCullough JA, et al. Component separation supported by Strattice mesh in the repair of contaminated ventral hernias. Colorectal Disease. The Association of Coloproctology of Great Britain and Ireland. 13(Suppl. 6), 28-62, P253
STRATTICE™ Tissue Matrix has been developed to strengthen tissue where there is weakness.
Preclinical studies have shown that STRATTICE™ Tissue Matrix regenerates to the recipient’s own tissue through cell repopulation, white cell migration and rapid revascularization.**(1)
Clinically proven: publications in more than 90 peer-reviewed articles on 2,000 hernia patients.*
Strattice™ is unique because of the way it is created. It uses a proprietary tissue processing technique, optimizing Strattice Tissue Matrix as a viable, intact regeneration base.
References:
- * Searches performed on PubMed, Google, Google Scholar and ScienceDirect® in June 2016.
- ** Correlation of these results to results in humans is not established.
- Sun WQ, Xu H, Sandor M, Lombardi J. Process-induced extracellular matrix alterations affect the mechanisms of soft tissue repair and regeneration. Journal of Tissue Engineering. 2013;4.
Articles
- PROSPECTIVE STUDY OF SINGLE-STAGE REPAIR OF CONTAMINATED HERNIAS USING A BIOLOGIC PORCINE TISSUE MATRIX
- OUTCOME OF ABDOMINAL WALL HERNIA REPAIR WITH BIOLOGICAL MESH: PERMACOL™ VERSUS STRATTICE™
- IMPROVED OUTCOMES IN THE MANAGEMENT OF HIGH-RISK INCISIONAL HERNIAS UTILIZING BIOLOGICAL MESH AND SOFT-TISSUE RECONSTRUCTION IN A SINGLE CENTER EXPERIENCE
- BIOLOGIC MESH IN VENTRAL HERNIA REPAIR, OUTCOMES, RECURRENCE, AND CHARGE ANALYSIS
- VENTRAL HERNIA: RETROSPECTIVE COST ANALYSIS OF PRIMARY REPAIR, REPAIR WITH SYNTHETIC MESH, AND REPAIR WITH ACELLULAR XENOGRAFT IMPLANT